Prescription Device Definition Fda . a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. Any hearing aid that does not satisfy the. The information on this page is current as of mar 22, 2024. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. definition of a medical device. this section specifies the labeling requirements for prescription hearing aids. (continued) recognized in the official national formulary, or the united states.
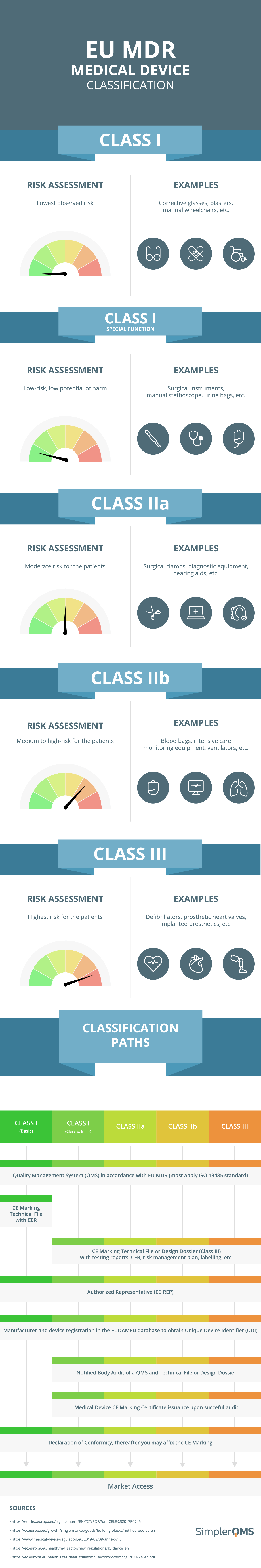
from www.simplerqms.com
(continued) recognized in the official national formulary, or the united states. this section specifies the labeling requirements for prescription hearing aids. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. Any hearing aid that does not satisfy the. The information on this page is current as of mar 22, 2024. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. definition of a medical device. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its.
Medical Device Classification (FDA & EU MDR) SimplerQMS
Prescription Device Definition Fda definition of a medical device. this section specifies the labeling requirements for prescription hearing aids. The information on this page is current as of mar 22, 2024. definition of a medical device. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. Any hearing aid that does not satisfy the. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. (continued) recognized in the official national formulary, or the united states.
From everythingmedschool.com
How To Write a Prescription (With Examples) Everything Med School Prescription Device Definition Fda prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. The information on this page is current as of mar 22, 2024. Any hearing aid. Prescription Device Definition Fda.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Prescription Device Definition Fda (continued) recognized in the official national formulary, or the united states. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. The information on this page is current as of mar 22, 2024. definition of a medical device. prescription devices a prescription device is,. Prescription Device Definition Fda.
From www.drugs.com
Ozempic FDA prescribing information, side effects and uses Prescription Device Definition Fda definition of a medical device. this section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. a device which, because of any potentiality for harmful effect,. Prescription Device Definition Fda.
From www.practicefusion.com
ePrescribing Electronic Prescription Software Practice Fusion Prescription Device Definition Fda a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. (continued) recognized in the official national formulary, or the united states. Any hearing aid that does not satisfy the. The information on this page is current as of mar 22, 2024. definition of a medical. Prescription Device Definition Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Prescription Device Definition Fda (continued) recognized in the official national formulary, or the united states. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. Any hearing aid that does not satisfy the. definition of a medical device. The information on this page is current as of mar 22, 2024. a. Prescription Device Definition Fda.
From www.medscape.com
Introduction to the New Prescription Drug Labeling by the FDA Page 2 Prescription Device Definition Fda a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. Any hearing aid that does not satisfy the. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. The information on this page is current. Prescription Device Definition Fda.
From www.goodrx.com
Prescribing Authority for Pharmacists Rules and Regulations by State Prescription Device Definition Fda Any hearing aid that does not satisfy the. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. this section specifies the labeling requirements. Prescription Device Definition Fda.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Prescription Device Definition Fda prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. (continued) recognized in the official national formulary, or the united states. Any hearing aid that does not satisfy the. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral. Prescription Device Definition Fda.
From www.studocu.com
Week 2 State Specific Guidelines for Prescribing Controlled Substances Prescription Device Definition Fda The information on this page is current as of mar 22, 2024. (continued) recognized in the official national formulary, or the united states. this section specifies the labeling requirements for prescription hearing aids. Any hearing aid that does not satisfy the. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of. Prescription Device Definition Fda.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Prescription Device Definition Fda this section specifies the labeling requirements for prescription hearing aids. The information on this page is current as of mar 22, 2024. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. prescription devices a prescription device is, by definition under 21 cfr 801.109,. Prescription Device Definition Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Prescription Device Definition Fda (continued) recognized in the official national formulary, or the united states. Any hearing aid that does not satisfy the. this section specifies the labeling requirements for prescription hearing aids. The information on this page is current as of mar 22, 2024. definition of a medical device. prescription devices a prescription device is, by definition under 21 cfr. Prescription Device Definition Fda.
From www.fda.gov
How Do I Use Prescription Drug Labeling FDA Prescription Device Definition Fda (continued) recognized in the official national formulary, or the united states. definition of a medical device. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary. Prescription Device Definition Fda.
From www.bloomberg.com
Trump’s FDA Might Greenlight DrugPrescribing Apps for Chronic Ailments Prescription Device Definition Fda definition of a medical device. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. (continued) recognized in the official national. Prescription Device Definition Fda.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Prescription Device Definition Fda this section specifies the labeling requirements for prescription hearing aids. prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. (continued) recognized in the official national formulary, or the united states. The information on this page is current as of mar 22, 2024. definition of a medical. Prescription Device Definition Fda.
From www.slideserve.com
PPT CIRCULATORY SYSTEM DEVICES PANEL Thursday, July 29, 2004 Prescription Device Definition Fda a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. definition of a medical device. (continued) recognized in the official national formulary, or the united states. a device which, because of any potentiality for harmful effect, or the method of its use, or the. Prescription Device Definition Fda.
From watsonshealth.com.ph
PRESCRIPTION MEDICATIONS Prescription Device Definition Fda a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. Any hearing aid that does not satisfy the. prescription devices a. Prescription Device Definition Fda.
From emmainternational.com
Discovering FDALabel Your GoTo Labelling Tool Prescription Device Definition Fda prescription devices a prescription device is, by definition under 21 cfr 801.109, a device which, because of any potentiality for. The information on this page is current as of mar 22, 2024. definition of a medical device. Any hearing aid that does not satisfy the. a device which, because of any potentiality for harmful effect, or the. Prescription Device Definition Fda.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Prescription Device Definition Fda Any hearing aid that does not satisfy the. (continued) recognized in the official national formulary, or the united states. a device which, because of any potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its. a device which, because of any potentiality for harmful effect, or the method of its use,. Prescription Device Definition Fda.